A Public Health Alert
In July 2025, the UK Health Security Agency (UKHSA) reported 38 confirmed cases of iatrogenic botulism linked to cosmetic injections occurring between June 4 and July 14 in the North East, East Midlands, and East of England regions. Symptoms ranged from difficulty swallowing and speech slurring to respiratory problems requiring support. Investigations point to unlicensed Botox-like products as the potential cause, prompting urgent health warnings. UKHSA cautioned individuals seeking aesthetic treatments to only use licensed products, prescribed via a face-to-face consultation with a qualified medical prescriber, and administered by properly trained professionals.
Why Such Risks Persist
⚖️ Regulatory Gaps & Unqualified Practitioners
Despite clear MHRA regulations that Botox is a prescription-only medicine, multiple reports show that thousands of illegal injections are still being performed in unregulated settings such as salons, private homes, hotel rooms, and even public toilets thesun.co.uk. Many operators advertise Botox or fillers online without any medical oversight—which is plain illegal. In Scotland, for example, the number of complaints about rogue beauticians performing injectable treatments soared from 121 in 2019 to 303 in 2023, with some providing injections after only a day’s training, leading to serious complications and disfigurement.
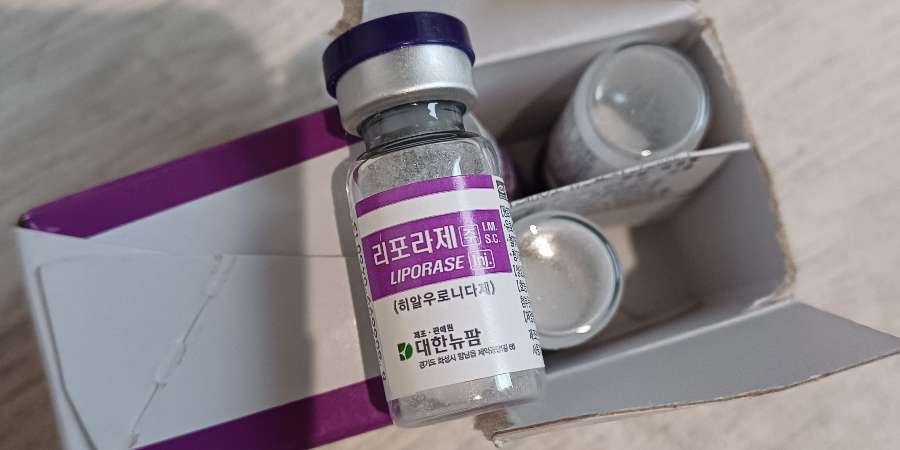
🧴 Fake or Unlicensed Products
Local authorities have seized batches of unapproved Botox-like products—often imported from overseas or purchased online—that lack proper labelling and quality controls. Consumers have been exposed to serious harms, including permanent scarring and disfigurement.
🚫 Remote Prescribing & Insufficient Consultation
Save Face highlighted widespread non‑compliance with prescribing rules. Investigations revealed some medics issuing Botox prescriptions remotely—without face-to-face consultation—despite professional guidelines (by GMC and NMC) requiring in-person assessment due to safety concerns. In many reported incidents, patients said they never even signed a consent form.
What the UKHSA Report Tells Us
The UKHSA’s warning confirmed that botulism cases traced to cosmetic injections involve unregulated products, likely containing improperly stored or unlicensed toxins. The agency stressed that symptoms may take up to four weeks to appear, and urged anyone who develops neurological signs after Botox to seek urgent medical attention.
This outbreak underlines a growing public health threat—not biological in origin—but due to cheap, unlicensed products administered by untrained individuals.
Save Face: Setting Standards in a Wild West Industry
 Save Face is the UK’s government-recognised register of accredited practitioners in non-surgical aesthetics. They vet individuals for appropriate medical qualifications, training, insurance, and adherence to safe prescribing practices.
Save Face is the UK’s government-recognised register of accredited practitioners in non-surgical aesthetics. They vet individuals for appropriate medical qualifications, training, insurance, and adherence to safe prescribing practices.
- In 2020, they received 934 Botox-related complaints, many involving providers who failed to deliver face-to-face consultations or explain risks properly saveface.co.uk.
- Save Face actively campaigns to raise public awareness and enforce safe practices in the industry, collaborating with media investigations such as The Botox Bust on BBC Three.
💬 Why This Happens: Root Causes
- Lack of Licensing & Oversight
Until regulation is implemented across the UK, anyone can legally perform injectables regardless of medical training, leading to unqualified practitioners operating unchecked.
- Commercial Pressure & Misleading Advertising
Affordable “Botox parties,” social media influencers, and clinics offering bargain treatments lure vulnerable customers who don’t know the risks - Easy Access to Illegal Products
Unlicensed Botox products are sold online or via informal supply chains, bypassing MHRA safety and quality controls—spreading botulism and infection risk GOV.UK. - Inadequate Prescribing Practices
Some prescribers bypass legal safeguards by issuing prescriptions remotely or based on minimal screening, ignoring the standards expected by regulatory bodies.
🔐 How Clients Can Protect Themselves
1. Choose Accredited Practitioners
Stick to professionals registered with Save Face or JCCP (Joint Council for Cosmetic Practitioners)—both accredited by the PSA
Ensure the product is licensed in the UK—only use brands such as Botox®, Azzalure®, Bocouture®, Alluzience®, Letybo®, or Nuceiva®. Beware of unfamiliar names like Botulax, reNTox, or Innotox
Ensure a medical professional (doctor, nurse, dentist, pharmacist) personally assesses you before prescribing Botox. No remote prescriptions should be accepted
Ask to see proof of medical qualifications, full aesthetics training (ideally Level 7), and insurance for complications.
5. Documentation & Consent
You should receive a full written consent form detailing risks, alternatives, aftercare, and legal rights. Lack of consent form or medical record keeping is a red flag.
Avoid low-cost offers with no medical facilities, no consultation, and no product transparency. If something feels too cheap or rushed—walk away.
Final Thoughts: Safety Matters More Than Savings
The UK botulism outbreak is a stark reminder that cosmetic enhancements pose real medical risks, particularly when carried out outside regulations. Botulism may be rare, but it’s severely life‑threatening—and entirely preventable.
By choosing regulated, accredited practitioners and insisting on licensed products and proper medical protocols, clients can protect themselves from serious harm. Save Face and JCCP represent your best safeguard. And until formal licensing is introduced, ensuring face-to-face consultations and full disclosure is your strongest defence.
Together, we can push the industry toward safer standards—because your health always comes first.
Further reading
UK health officials issue warning over cosmetic jabs after 38 botulism cases